Tissues in the adult organism contain a population of tissue-specific stem cells that provide the cellular basis for homeostatic maintenance of adult tissues. Our goal is to elucidate the molecular mechanisms influencing the fate of normal and transformed adult stem cells in the intestine and haematopoietic system.
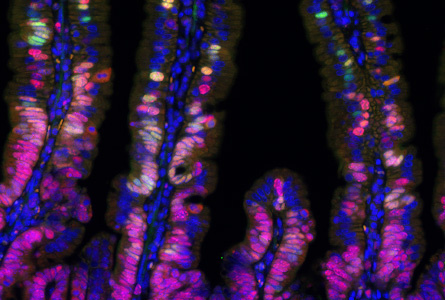
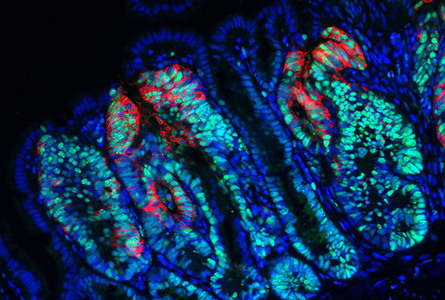
Since the fate of intestinal stem cells is determined by the Wnt signalling pathway, one of our aims is to find and characterize genes that are regulated by Wnt signalling. It is presumed that the first oncogenic mutation provides selective advantage to the prospective cancer cells, which multiply and form the initial neoplastic lesion. Interestingly, our results indicate that depending on the position in the intestine, the transformed cell activates a specific transcriptional programme to ensure its long-term survival in the tissue. Importantly, only a cell that stays in the body for a long time then might accumulate additional alterations that drive tumour growth and progression. The identity of somatic stem cells is determined by a specific microenvironment, so-called stem cell niche, which promotes a strict control of tissue homeostasis. Recently, we have discovered that sub-epithelial mesenchymal cells constitute the intestinal stem cell niche by secreting Wnt ligands that promote the stem cell renewal. We characterize other specific features of niche cells.
Our research interest also includes the molecular bases of haematological diseases, especially disorders related to the production and renewal of red blood cells. Myeloproliferative neoplasms (MPN) represent a group of disorders arising due to the genetic defect(s) in haematopoietic stem cells. Our aim is to identify the new genetic predispositions to MPN and define their effect(s) in various cellular backgrounds. We demonstrated that germ-line (or acquired) mutations in the gene encoding Janus kinase 2 (JAK2) enhances oncogenic JAK2/STAT signalling and causes a specific clinical course of the disease in MPN patients.
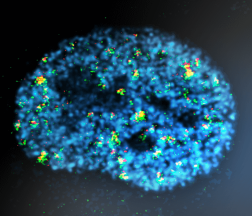
We employ genetically modified (transgenic, gene knockout or knock-in) mice as the main experimental tool. Beside in vivo models, we also use intestinal organoid cultures derived from healthy or tumour tissue. To study the genetic bases of haematological malignancies, we perform next-generation sequencing of genomic DNA isolated from human specimens.