
| Location | IMG – building F 1st floor – rooms 1.60, 1.52 basement – rooms 01.155.1, 01.150, 01.152, 01.153, 01.154, 01.151 |
| Phone | +420 241 063 153 |
| Documents | How to properly acknowledge the core facility in publications |
The Electron Microscopy Core Facility of the Institute of Molecular Genetics of the Czech Academy of Sciences (EMCF IMG) provides state-of-the art equipment and expertise for a broad range of sample preparation and ultrastructural imaging techniques for cell biology and structural biology. The core facility staff has experience with various kinds of biological samples: human and animal cell cultures, plant and animal tissues, worms, insects, microorganisms, lipid micelles, nanoparticles, protein molecules or crystals. We provide an open non-discriminatory access to our technologies via Czech-BioImaging and Euro-BioImaging infrastructures. To gain access to the EMCF IMG services, new users can use Czech-Bioimaging access, Euro-Bioimaging access, or contact us directly (vlada@img.cas.cz).
As the spectrum of approaches and workflows in electron microscopy is very wide, we help the users to select an appropriate technique and to plan the whole experiment. The sample preparation, imaging, and data processing can be done fully by facility staff, or we can provide sufficient training and tailored support for independent use of the technologies and equipment. We organize a yearly one-week practical course of transmission electron microscopy in life sciences for beginners and intermediate users.
The core facility is equipped with two transmission electron microscopes (TEM) – a standard instrument for routine observation and an advanced 200 kV instrument providing the possibility of high-resolution TEM, STEM, 3D electron tomography, cryo-electron microscopy and EDS elemental analysis and mapping. A fluorescence cryoCLEM microscope enables correlation of light and electron microscopy data for cryo-samples. Additionally, a cryoFIB-SEM microscope TESCAN AmerCryo is available in the frame of collaborative project with TESCAN company.
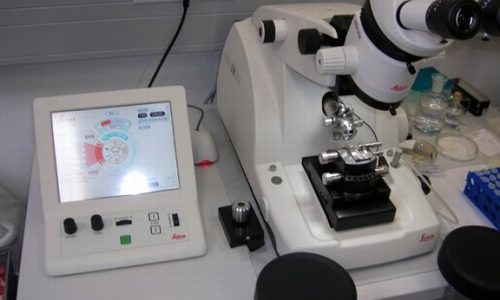
Sample preparation techniques include both standard and advanced methods, such as chemical fixation and resin embedding, cryofixation using high-pressure freezing technique or plunge-freezing, freeze-substitution, (cryo)sectioning, freeze-fracture replica, immunolabeling, critical-point drying and various workflows for correlative light and electron microscopy (CLEM). High-pressure freezing machines, two automatic freeze-substitution machines, freeze-fracture and replica making device, (cryo)ultramicrotomes, Leica EM GP2 for automated plunge-freezing, cryoCLEM light microscope, as well as additional wet lab equipment are available.
Our team has a long expertise in the development and optimization of various methods in biological electron microscopy, both in the frame of user projects and in collaboration with companies – manufacturers of microscopy equipment. Recent advances include a range of cryo-workflows utilizing frozen hydrated FIB-milled lamella with subsequent cryo-electron tomography or cryo-EDS chemical analysis. For statistical analysis of immunolabeling in electron microscopy, we developed an on-line tool Pattern.
Since 2016, EMCF IMG is involved in the national research infrastructure for biological and medical imaging – Czech-BioImaging. It is a distributed infrastructure of leading imaging facilities in the Czech Republic, providing open access to technologies and expertise to users from Czech Republic and from abroad. The EMCF IMG is part of the Prague Node of Czech-BioImaging, and since 2020, a part of Prague Node of the European Research Infrastructure Consortium Euro-BioImaging ERIC.





The Electron Microscopy core facility is supported from the program for large research infrastructures of the Ministry of Education, Youth and Sports within the project – National Infrastructure for Biological and Medical Imaging (Czech-BioImaging – LM2018129, LM2023050).
The modernization of equipment was supported by OP RDE (CZ.02.1.01/0.0/0.0/16_013/0001775, CZ.02.1.01/0.0/0.0/18_046/0016045 – Modernization and support of research activities of the national infrastructure for biological and medical imaging Czech-BioImaging).





