Retroviruses, endogenous retroviruses, receptors for retrovirus entry, restriction factors, antivirus innate immunity, gene editing in chicken, syncytins, epigenetic suppression of retroviruses
Replication of retroviruses in host cells is a result of the interplay between the virus and multiple cellular factors, either the virus dependence factors necessary for subsequent steps of the replication cycle or restriction factors that block virus replication and contribute to the innate antiviral immunity. The major focus of our group are receptors for retroviruses that specifically attach the virus and assist in virus entry. Avian leukosis virus (ALV), an important pathogen in the domestic chicken, diversified into several subgroups differing in their receptor usage. This creates an opportunity to study the virus-host coevolution and accommodation of the virus to a new host (1). As a practical outcome of this research, we pioneer the techniques of CRISPR/Cas9 genome editing of the chicken (together with partners from commercial sphere) and manipulate the genes for ALV receptors with the aim to obtain virus-resistant chicken lines (2).
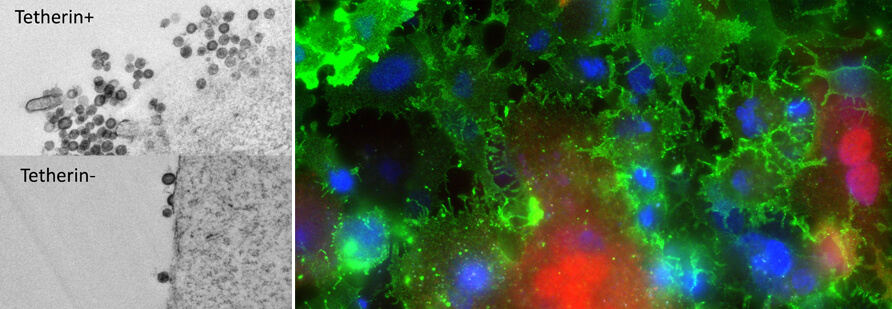
Working with avian retroviruses, we also need a strong background in the genetics and genomics of birds, particularly the chicken. Our analyses of the GC-rich parts of the chicken genome (4) led to the discovery of several genes formerly considered to have been lost in the lineage of galliform birds (TNF-a, BST-2, PTX3, EPO, EPOR, etc). In combination with CRISPR/Cas9 gene editing either in cell lines or in vivo, we are now able to test the biological activities of these genes and compare them with functions in other vertebrates. For example, chicken BST-2/Tetherin exerts antiviral activity against ALV (Fig. 1 on the left) and potentially against other enveloped viruses (avian flu, avian coronaviruses, etc.). Factors of innate immunity will be of particular interest in the future.
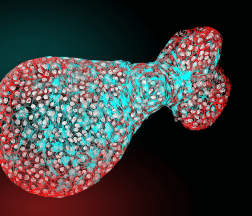
We are also interested in endogenous retroviruses in the genome of vertebrates, which are the signs of ancient infections. We identified endogenous lentiviruses and deltaretroviruses in various mammalian lineages – the oldest HIV- and HTLV-related molecular fossils. Endogenous retroviruses adopt unexpected functions like syncytin-1, which is inevitable for cell-to-cell fusion in human placenta (Fig. 2 on the right). We focus on the receptor for syncytin-1 (hASCT2 amino acid transporter) and epigenetic regulation of syncytin-1 expression (5), which occurs physiologically in the placenta and aberrantly in germline tumours. Epigenetic regulation has been shown previously in HIV-1 infection, where the persistence of CpG-methylated and transcriptionally silent proviruses is the major obstacle to functional cure. As the provirus silencing strongly depends on the site of integration and its epigenetic features, we also perform genome-wide analyses of retrovirus integration preference and distribution of proviruses.